DHFR Catalytic Action
| |||||
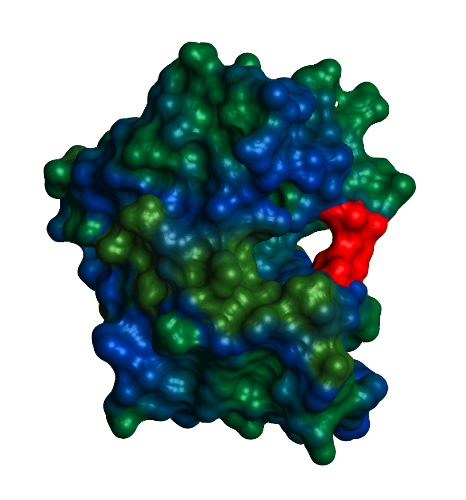
Because of DHFR's clinical importance, there have been many studies on the enzyme's catalytic function and changes in protein dynamics in response to ligand binding. The enzyme's catalytic action proceeds as follows: NADPH and DHF both bind the active site. DHFR wraps its sidechains around the two ligands, positioning them tightly next to one another. Then, the enzyme transfers hydrogen atoms from NADPH to DHF to yield the final product, THF. After protonation and hydride transfer, NADP+ leaves first and another NADPH is added resulting in a DHFR-THF-NADPH complex. Then, THF is released and the DHFR-NADPH complex is able to carry out another round of catalysis. An extremely flexible protein, DHFR undergoes many conformational changes throughout the course of catalysis. The subdomains are able to shift and rotate to facilitate proper ligand binding and the three active site loops play a vital role. The Met20 loop (shown in red) lies directly over the active site, protecting it from solvent, and is responsible for regulating access to the active site. Under physiological conditions, the Met20 loop can assume one of two characteristic conformations, occluded or closed. The conformation switches from occluded to closed during the step in which THF is released, which is also the rate determining step. The F-G and G-H loops hydrogen bond with the Met20 loop, stabilizing the various conformations. The availability of the active site loops depends on the ligands bound. DHFR assumes the occluded conformation when DHF is bound. Binding of NADPH leads to the formation of the closed conformation, in which the Met20 loop hydrogen bonds with the nicotinamide ring of NADPH. This closes the active site, protecting it from solvent, and it allows the close positioning of the substrate and cofactor. The difference between the occluded and closed conformations is the position and hydrogen bonding between the Met20 loop and the F-G and G-H loops. In the occluded conformation, there is hydrogen bonding between the Met20 loop and the G-H loop. In the closed conformation, new hydrogen bonds form between the F-G loop and the Met20 loop. | |||||
|
Previous: Introduction |